
Information Center
Your Health. Your Family. Your Choice.
 |
National Vaccine Information Center Your Health. Your Family. Your Choice. |
The MedAlerts Blog |
October VAERS data is out and it has a few surprises.
Why, when this winter's Flu season is in full swing, are we still learning about the last Flu season? The answer has two parts: (1) the new reports are not from the US and (2) they took a long time to get here.
Each of these new reports states in its write-up that it was sent by the manufacturer on August 25 of this year and involves a patient in a "foreign country" (but does not say which). You can find these reports for yourself by searching for "25 August 2010" in the Write-up Symptom field.
What can we learn about these reports? I wanted to find out when these vaccinations were given so I made a graph of the vaccination dates, by month. On the left is a graph of the new reports and on the right is a graph of the previous H1N1 data.
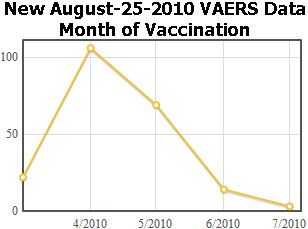
The obvious explanation for the half-year shift is that these reports come from a country below the equator, where the winter Flu season is in April. That means the data comes from Australia, New Zealand, or somewhere in Africa, or South America.
But one question remains: why, if the data was sent on August 25, did it take this long to appear in VAERS?
Every VAERS report has date information telling when it was submitted to VAERS and when it actually appeared in VAERS. It would make sense if the submission date of these new reports was in August or September. However, only one of these reports was submitted in August and just 67 of them in September. The bulk of them (149) were not submitted until October. Who knows whether more will be submitted in November.
Why the delay? It could be pointed-out that VAERS, which is chartered with releasing this data, is also chartered with protecting the identity of the people involved. This "sanitizing" of the data to hide identification probably takes some time. However, most drug companies that collect and submit data to VAERS do a pretty thorough job of sanitizing the data themselves. In fact, the typical VAERS report that comes from a pharmaceutical corporation has so much of the data removed that it is less useful for normal analysis (for example, the state in which the patient lives is often missing).
Regardless of whether or not the full story has been told, this new data from a southern-hemisphere country shows that the H1N1 Flu Vaccine left a wide swath of damage in its path.
<< 9/2010: Flu season preview 11/2010: H1N1 Flu and miscarriages >>
Copyright ©
2026 National Vaccine Information Center. All rights reserved.
21525 Ridgetop Circle, Suite 100, Sterling, VA 20166